
Knowing how cleaners compare to one another is important. Whether it is “do no harm” cleaners that are being compared with each other, or it is do no harm cleaners being compared to more harmful cleaners. Cleaners can be or are complicated things to understand and choose. They are made in labs by chemists from a multitude of ingredients and ingredient proportions and combinations. This can be further complicated by manufacturer claims and the marketing of those claims. And sorting through their actual makeup or content vs their claims and marketing is difficult.
We are going to attempt to separate the facts from the claims and dig as deeply into any given cleaner as is possible by examining its true makeup. By examining its ingredients, ingredient proportions, and ingredient combinations, we can better understand its true effects and the consequences of these effects. We will begin with some explanations, terms, and definitions of the science and chemistry behind how cleaners are made and measured.
We feel this very brief science address concerning chemistry is important as a start to a better understanding. And because many of these terms are often bandied around, we also wanted to do what we could to present them with definitions. We hope to refine and define this portion as we collect more knowledge. Below this portion is where you will find the cleaners we are currently examining.
Cleaning…A Better Understanding Of What Makes For A Do No Harm Cleaner

What Is pH and Why Is It Important?
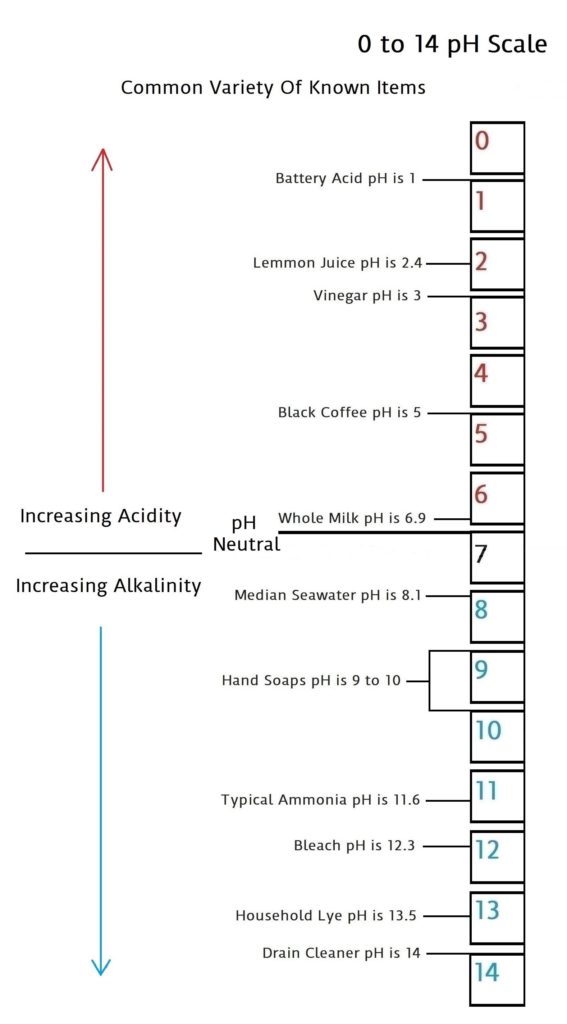
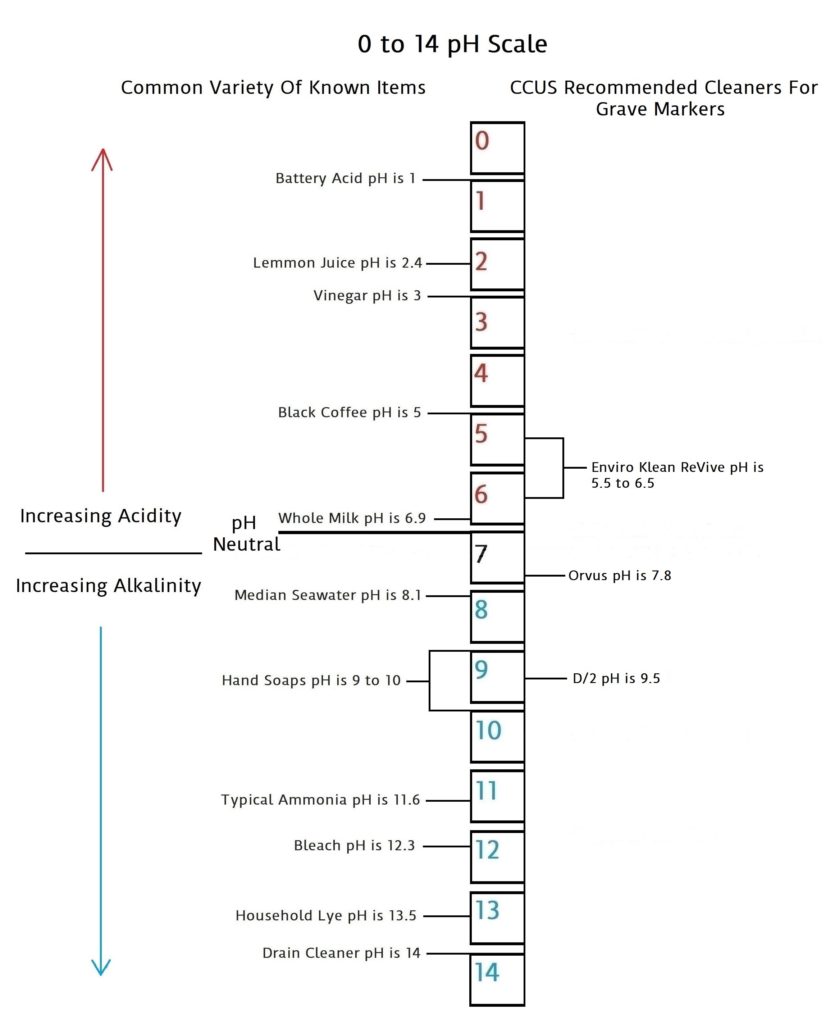
pH is a scale of 0 to 14 that measures acidic quantities and qualities against alkaline quantities and qualities. A pH less than 7 is said to be acidic and solutions with a pH greater than 7 are basic or alkaline. pH neutral is 7. Human pH neutral is 7.30 to 7.45 slightly alkaline. Being as close to pH neutral is important because this is the least caustic area of the scale between the two extremes.
This scale will give you an idea of how pH is measured using common items you may be familiar with.
Acidic and basic are two extremes that describe a chemical property of chemicals. Mixing acids and bases can cancel out or neutralize their extreme effects. A substance that is neither acidic nor basic is neutral.
The pH scale measures how acidic or basic a substance is. The pH scale ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic. A pH greater than 7 is basic.
The pH scale is logarithmic and as a result, each whole pH value below 7 is ten times more acidic than the next higher value. For example, pH 4 is ten times more acidic than pH 5 and 100 times (10 times 10) more acidic than pH 6. The same holds true for pH values above 7, each of which is ten times more alkaline (another way to say basic) than the next lower whole value. For example, pH 10 is ten times more alkaline than pH 9 and 100 times (10 times 10) more alkaline than pH 8.
Pure water is neutral. But when chemicals are mixed with water, the mixture can become either acidic or basic. Examples of acidic substances are vinegar and lemon juice. Lye, milk of magnesia, and ammonia are examples of basic substances.
pH Scale Terms
Acidity
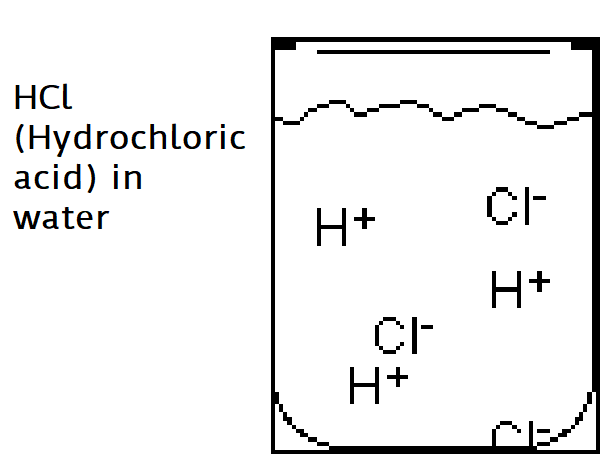
Acids are ionic compounds (a compound with a positive or negative charge) that break apart in water to form a hydrogen ion (H+).
The strength of an acid is based on the concentration of H+ ions in the solution. The more H+ the stronger the acid.
Example: HCl (Hydrochloric acid) in water
Characteristics of Acids:
Acids taste sour
Acids react strongly with metals (Zn + HCl)
Strong Acids are dangerous and can burn your skin
Examples of Acids:
- Vinegar
- Stomach Acid (HCl)
- Citrus Fruits
Bases or Alkaline
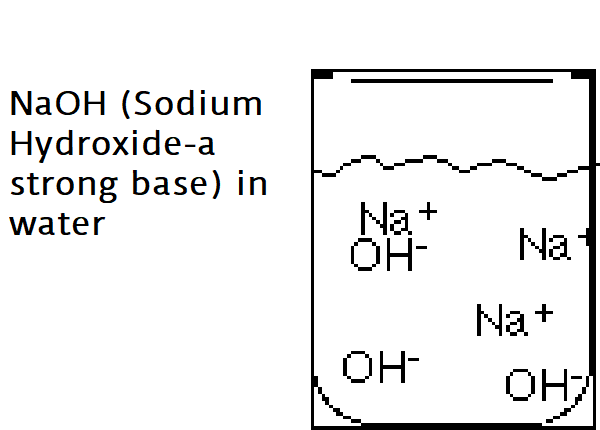
Bases are ionic compounds that break apart to form a negatively charged hydroxide ion (OH–) in water.
The strength of a base is determined by the concentration of Hydroxide ions (OH–). The greater the concentration of OH– ions the stronger the base.
Example: NaOH (Sodium Hydroxide-a strong base) in water
Solutions containing bases are often called alkaline.
Characteristics of Bases:
Bases taste bitter
Bases feel slippery
Strong bases are very dangerous and can burn your skin
Examples:
- Lye (Sodium Hydroxide)
- Ammonia
Neutralization Reactions
When acids and bases are added to each other they react to neutralize each other if an equal number of hydrogen and hydroxide ions are present.
When this reaction occurs -salt and water are formed.
HCl + NaOH ⇒ NaCl + H2O
(Acid) (Base)— (Salt) (Water)
What are some useful applications of this reaction?
pH Indicators
Indicators– An indicator is a special type of compound that changes color as the pH of a solution changes, thus telling us the pH of the solution.
Conclusion
pH values are very important in determining the severity of any given cleaner that is applied to a grave marker as well as to your health and safety. As you can see from the scale, the further you get to either end of the scale, the more caustic the product becomes. And there for the more risk of damage to the grave marker, yourself, and the environment. The safest and best products will be the ones closest to the pH neutral middle. But, keep in mind the increase that occurs in both directions of the scale as it moves up one notch in either direction, and how this multiplies 10 and 100 fold. It is also good to note a bit of common sense philosophy by not trying to possibly compare what you as a human may be able to ingest vs what the grave marker may be able to ingest. The human stomach can ingest things from lemon juice at a 2.4 to milk of Magnesia at 10.5 because it has its own gastric acids that are between 1.5 and 3.5. That’s in the neighborhood of battery acid to vinegar.
The Science And Terms Behind What Make Up Cleaners
Ionic and Nonionic Detergents
Ionic…
The polar head group of ionic detergents contain either a positive (cationic) or negative (anionic) charge. Anionic detergents typically have negatively-charged sulfate groups as the hydrophilic head; whereas cationic detergents contain a positively-charged ammonium group. They are frequently used for common household soaps and cleansers, but can also serve specific purposes in a lab setting. Ionic detergents have stronger effects than uncharged detergents, because they bind to protein molecules, altering the protein’s charge and structure. They should be used when the preferred outcome involves modifying proteins and disrupting cellular structures, such as in gel electrophoresis.

Nonionic…
Non-ionic detergents contain molecules with head groups that are uncharged. They can be further classified into two types: polyoxyethylene (and related detergents), and glycosidic compounds. Detergents based on polyoxyethylene and similar compounds contain a neutral, polar head group and the tails are composed of hydrophobic oxyethylene chains, or, in some cases, ethylene glycoether chains. Detergents with a glycosidic base tend to use a sugar as the head group, such as glucose or maltose, and contain an alkyl polymer tail. Non-ionic detergents are less harsh than ionic detergents, having a limited ability to break protein-protein interactions. Because of this, they are considered to be non denaturing and are great for processes where keeping protein structure intact is important. These detergents are effective at isolating active membrane proteins, or breaking lipid-lipid and lipid-protein interactions.
Conclusion
The above statements are the differences between ionic and nonionic detergents. Through our own research, we have not yet been able to determine the effects created onto stone by ionic detergents, but the NCPTT has said to use nonionic, which is the direction we’ve been following. Until then, all that we’ve been able to find is that ionic detergents are harsher than nonionic detergents. Unfortunately, “harsh” is a relative term, and what may be harsh to your skin may be fine on stone, and vice versa. We are by no means trying to prove wrong what is considered to be common knowledge in the field. On the contrary, we’re trying to support it, but the information is scarce and we are continuing to dig. Until we are able to find sufficient information, we will continue to support the use of non-ionic cleaners in the field of stone restoration.

Surfactant…
Surfactants are compounds that lower the surface tension (or interfacial tension) between two liquids, between a gas and a liquid or between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, and dispersants.
Biocide…
A substance (such as an algicide or fungicide) that destroys or inhibits the growth or activity of living organisms. Source…Merriam-Webster.
Chelate…
Any of a class of coordination or complex compounds consisting of a central metal atom attached to a large molecule, called a ligand, in a cyclic or ring structure. Chelates are more stable than nonchelated compounds of comparable composition, and the more extensive the chelation—that is, the larger the number of ring closures to a metal atom—the more stable the compound. This phenomenon is called the chelate effect; it is generally attributed to an increase in the thermodynamic quantity called entropy that accompanies chelation. The stability of a chelate is also related to the number of atoms in the chelate ring. In general, chelates containing five- or six-membered rings are more stable than chelates with four-, seven-, or eight-membered rings. Source…Encyclopedia Britannica.
Quaternary Ammonium Compounds…
Any of numerous strong bases and their salts derived from ammonium by replacement of the hydrogen atoms with organic radicals and important especially as surface-active agents, disinfectants, and drugs. Source…Merriam-Webster.
Sodium Lauryl Sulfate…
A soaplike compound that lathers easily, used chiefly in laundry detergents, cleaning products, and toiletries such as shower gel and shampoo. Chemical formula: CH₃(CH₂)₁₁SO₄Na. Origin – From sodium + lauryl, an alcohol with twelve carbon atoms from which the compound is prepared, + sulphate. Source Oxford Dictionary.
Conclusion
We are not chemists with a brief working knowledge of cemetery conservatorship. We are cemetery conservators with a brief working knowledge of chemistry. And thus being the case, we will further pursue those with the knowledge to help us better understand and bridge this gap to become more well-rounded on this subject.
A Look At Biological Cleaners
The following is a condensed version of the scientific lab reports compiled on the study of D/2, in combination with information and comparisons of another biological cleaner by the NCPTT. The CCUS has them both of these biocides on our list of recommended biological cleaners.
The study of D/2 Biological Solution from 2004 to 2011 in conjunction with NCPTT studies and findings
NCPTT’s Mary Striegel reported the results of the six-year VA-funded study to Steve Muro, the VA Under Secretary for Memorial Affairs, and a variety of National Cemetery Administration officials. Based on NCPTT research, the U.S. Department of Veterans Affairs will implement new policies that ban bleach-containing cleaners and encourage the use of gentle biocidal cleaners for regular maintenance of more than three million headstones nationwide. The results of the study led NCPTT to develop a document on the best practice for cleaning government-issued marble headstones
The research which led to these recommendations included field and laboratory studies that cut across disciplines from chemistry and biology to materials science and conservation treatment development. There were two main goals of the study. The first goal was to find effective commercial cleaners that removed soiling and microorganisms which alter the appearance and degrade headstones. The second goal was to look at factors that led to the re-growth of microorganisms on the stone.
NCPTT researchers studied five different cleaners which can be easily applied in the field. The cleaners needed to be effective in improving the appearance of the headstone and do no harm to the marble. In the field, NCPTT evaluated cleaners on stones located at five different climates and in both sunny and shady environments. Microbiologists at Harvard’s School of Engineering and Applied Sciences evaluated the microorganisms originally on the stone including bacteria, fungi, and algae. They helped to follow the re-growth on the headstones after cleaning over an eighteen-month time period. Additionally, they conducted accelerated laboratory tests using fungi to distinguish between the best field performing cleaners.
The main recommendations include the following:
- Cleaning should be undertaken with the mildest, least-abrasive method.
- A biocidal cleaner performed the best in this study. Recommended biocidal cleaners include D/2 Biological Solution (which was tested in this study) manufactured by Sunshine Makers, Enviro Klean® BioWash®, or other cleaners that contain quaternary ammonium compounds.
- Soak the stone liberally with water before applying the cleaner with a hand or backpack sprayer or garden hose.
- Always keep the stone wet during cleaning and thoroughly rinse afterwards.
- Agitate the surface gently in a circular motion using a soft bristle brush. Clean small areas from the bottom up.
- Remember to rinse after cleaning each area and to thoroughly rinse the stone at the end to make sure that no cleaner is left behind.
Conclusion
The rigors described above that these cleaners were put through is absolutely true. Two independent labs were chosen and Harvard was chosen as a third lab to look over the findings of these two labs. The sixth recommendation is most likely one of a precautionary nature due to possible legality issues between a governmental agency and a manufacturer. The CCUS prefers to adhere to the manufacturer’s guidelines for use pertaining to leaving D/2 on the stone and not thoroughly rinsing it off. The CCUS and the majority of its conservators through repeated sustained use of this product can find no adverse effect by this, but find it produces better results in a more economical manner. You will find more specifics via our six standard questions under SOURCES…Pro Vs Con Results.
CCUS Recommended “Do No Harm” Cleaners On The pH Scale
We will add more information on biocide types and other cleaners for grave markers as it becomes available in this section. Below is a pH scale that gives pH balances for the cleaners we can recommend based on the best information we have been able to collect at the present time.
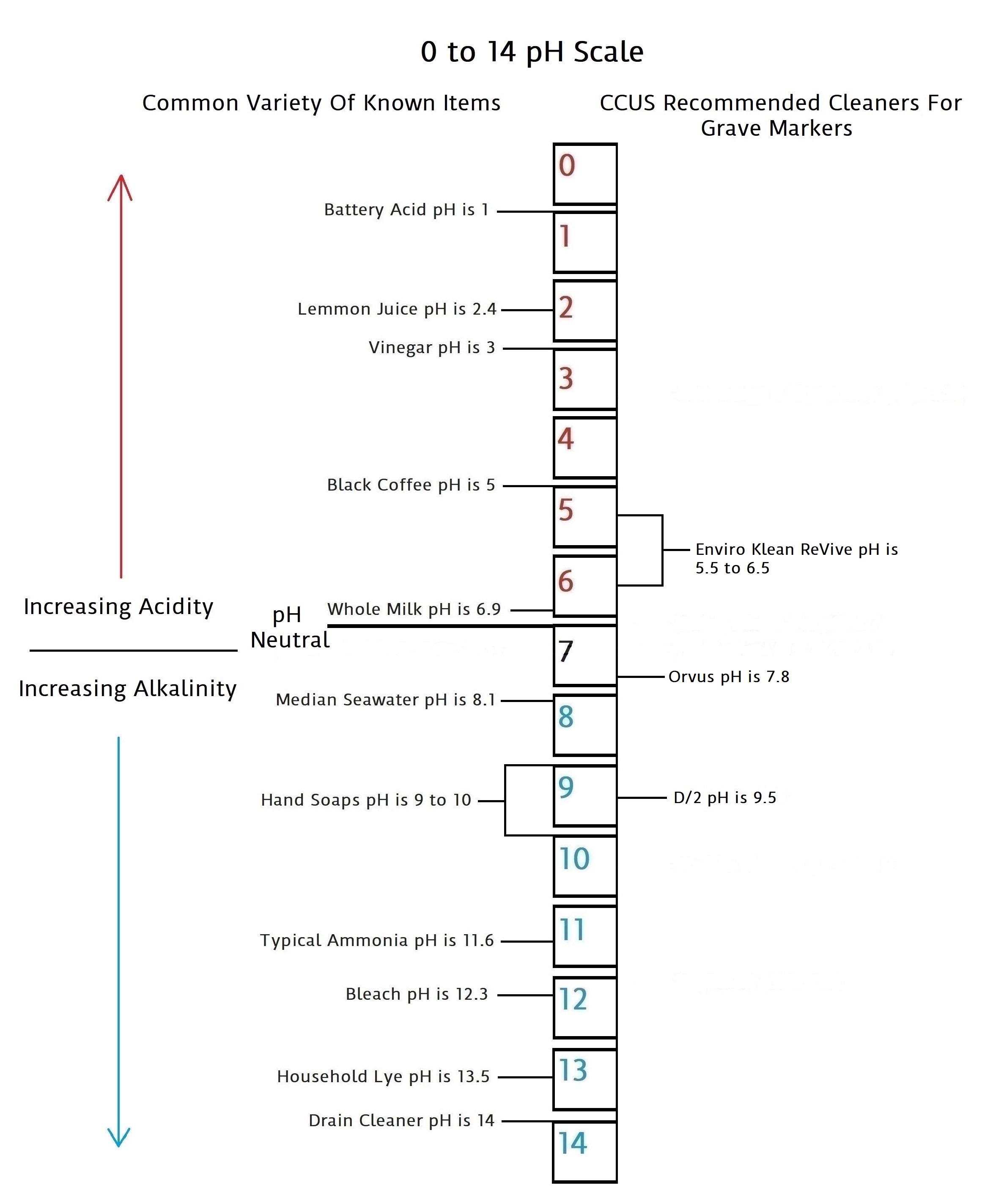
Enviro Klean ReVive…A Biocide Cleaner
Enviro Klean ReVive is a cleaner manufactured by PROSOCO, a company dealing in maintaining stone since 1939. This cleaner is in the top two most recognized and recommended biocides among cemetery conservators and people in the field of stone preservation. This combined with it being recognized by the NCPTT is why it is on our list of recommended cleaners. We hope to have more in-depth information that sheds greater light on its actual makeup and history in the near future. Coming soon, more specifics via our six standard questions under SOURCES…Pro Vs Con Results.
Conclusion
We have no other conclusion about this product than what we have just stated.
Orvus Paste…A Gentle All-Around Cleaner
Orvus is a sodium lauryl sulfate. It is completely biodegradable, non-ionic, and does not contain phosphates. Sodium lauryl sulfate is a “surfactant” – it removes stains and residues that are oily, so it’s found as an ingredient in lots of surface cleaners. It is versatile stuff and is used to clean vintage textiles, linens, needlework, and quilts. All things very prone to delicate dies and fibers that must be treated very gently.

“Orvus is a great mild detergent, it is safe for cleaning most anything including vintage textiles. It does work well on stone to remove general dirt and soiling. It will not kill, or remove biological growth like D2. So no harm will come from a soft bristle brush, Orvus, and a lot of water.”… (The NCPTT)
Ammonia…aggressive cleanings other half of the 1, 2, punch that damages grave markers
An article by Geologist Don Hilton and Cemetery Conservator Mark Morton
For years ammonia has been viewed as a safe and inexpensive cleaner for grave markers. And the golden rule has always been to use it at a ratio of 5 parts water to 1 part ammonia. And to never go above that ratio by increasing the amount of ammonia. Ammonia on its own is very alkaline on the pH scale, coming in at 11.6. To at least begin to understand the effects of ammonia that we are describing, we need to look at some basic chemistry and have a basic understanding of the importance of pH and pH neutral. The scale and description below should do just that.
What Is pH and Why Is It Important?
pH is a scale of 0 to 14 that measures acidic quantities and qualities against alkaline quantities and qualities. A pH less than 7 is said to be acidic and solutions with a pH greater than 7 are basic or alkaline. pH neutral is 7. Human pH neutral is 7.30 to 7.45 slightly alkaline. Being as close to pH neutral is important because this is the least caustic area of the scale between the two extremes.
This scale will give you an idea of how pH is measured using common items you may be familiar with.
Acidic and basic are two extremes that describe a chemical property of chemicals. Mixing acids and bases can cancel out or neutralize their extreme effects. A substance that is neither acidic nor basic is neutral.
The pH scale measures how acidic or basic a substance is. The pH scale ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic. A pH greater than 7 is basic.
The pH scale is logarithmic and as a result, each whole pH value below 7 is ten times more acidic than the next higher value. For example, pH 4 is ten times more acidic than pH 5 and 100 times (10 times 10) more acidic than pH 6. The same holds true for pH values above 7, each of which is ten times more alkaline (another way to say basic) than the next lower whole value. For example, pH 10 is ten times more alkaline than pH 9 and 100 times (10 times 10) more alkaline than pH 8.
Pure water is neutral. But when chemicals are mixed with water, the mixture can become either acidic or basic. Examples of acidic substances are vinegar and lemon juice. Lye, milk of magnesia, and ammonia are examples of basic substances.
Ammonia as an industrial fertilizer
Ammonia (NH3) is the foundation for the nitrogen (N) fertilizer industry. It can be directly applied to soil as a plant nutrient or converted into a variety of common N fertilizers, but this requires special safety and management precautions. Ammonia has the highest N content of any commercial fertilizer, making it a popular source of N despite the potential hazard it poses and the safety practices required to use it. For example, when NH3 fertilizer is applied directly to soil, it’s in a pressurized liquid that will immediately become vapor if exposed to air after leaving the tank. To prevent such releases into the atmosphere, growers use various tractor-drawn knives and shanks to place it at least 10 to 20 cm (4 to 8 inches) below the soil surface. Ammonia will then rapidly react with soil water to form ammonium (NH4+), which is retained on the soil cation exchange sites.
Ammonia as a simple fertilizer by adding water
Ammonia is sometimes dissolved in water to produce aqua ammonia, a popular liquid N fertilizer. Aqua ammonia doesn’t need to be injected as deeply as NH3, which provides benefits during field application and has fewer safety considerations. Aqua ammonia is frequently added to irrigation water and used in flooded soil conditions.
Summation: When you take household ammonia and add water, you are making a form of “liquid N fertilizer”. And even though this type of fertilizer does not need to be “injected deeply”, the porosity of stones like marble, sandstone, limestones, etc. will in a way, self-inject this like a sponge due to the stones natural porosity. Most all cleaning that occurs on grave markers is to remove or kill biological growth such as lichens and moss. Many of these biological growths attach themselves deep into the stone, making them very difficult to dislodge. So when you apply your homemade liquid N fertilizer in an effort to get rid of biological growth, you are actually fertilizing it at its core where it resides below the stones surface. If the stone already has a “protective crust or skin” as described above, it at least has some natural protection to begin with. But when you mechanically remove that layer as you spray down the stone with your water and ammonia solution, you are laying the stone bare to everything and greatly aiding by injecting this into the stone. This is why we refer to this as a 1, 2, punch that damages a porous stone forever.



Above left to right, are 3 white marble stones that were cleaned with the Nyalox wheel and ammonia. The first stone was already showing signs of natural dissolving, or as many conservators call it, “the melted candle wax effect”. The middle stone represents the average amount of damage that occurs. The stone on the right is an example of how the Nyalox wheel has ground off most of the delicate detailed carving, and is beginning to show some biological regrowth. All 3 of these stones were completely white directly after the Nyalox method was completed. You never know for sure the severity of damage that will occur. Cloverdale Cemetery, Cloverdale Ind.
In addition to this, you have opened up the stone to a plethora of elements natural & manmade. Let’s start with the natural elements. We have all of the biological growths that occur naturally in nature as the natural process that breaks down everything on earth. This is further accelerated as wind and rain leave soil deposits as part of this cycle. So you roughly have soil and seeds and seasonal growth patterns. Manmade elements can range from a wide variety of atmospheric pollutants and acid rains, to any sort of carry over used in the farming industry, from fertilizers to pesticides. So the grave marker becomes very much like a petri dish.
Preserving anything is always a fight against natural occurrences in nature. And the best way to combat this when it comes to grave markers is with the least invasive methods possible. It’s a matter of not going too far in an effort to make something look brand new, but to make a “do no harm” improvement. And realizing where the tipping point is between the two. And also realizing when something is as good as it will get or to leave it alone altogether.
SOURCES:
https://www.britannica.com/science/desert-varnish
https://pubs.usgs.gov/pp/1210/report.pdf
https://www.thoughtco.com/chemical-weathering-p2-4122736
Photo Courtesy…Mark Morton and Lee Creed, Images by Cemetery Conservators For United Standards
Conclusion
As far as pH balance is concerned, this product is very close to pH neutral. Orvus Paste has been recognized or recommended for a number of years by several organizations in the cemetery gravestone field as a safe cleaner to remove dirt and debris. From what we have been able to determine through available information, we are unable to find anything unsafe about it for grave marker cleaning. Besides its good pH rating, it is also no-ionic, which is another good factor to find in a cleaner. We will continue to look for further information that will allow us a closer examination of this product. You will find more specifics via our six standard questions under SOURCES…Pro Vs Con Results.

There has been much said, both negative and positive about this product for grave marker cleaning since it first came onto the scene a few years ago. This product has been quite popular among those looking for the quick, cheap, and easy to find solution for cleaning biological growth off of gravestones. Those in favor of this product site the previous reasons and back that up with the manufacturer’s claims.
Those not in favor of this product point out reasons not associated with easy button reasons of convenience and manufacturer’s claims. Their reasons are about being fact based due to rigorous testing and analysis in accordance with the “do no harm” practices for cemetery preservation and restoration. Their concerns are as follows.
Concern over this product having strong diverse chemical combinations that “may” damage old porous stones such as marble, sandstone, and other siltstones.
Concerns about an MSDS sheet with many warnings about safety precautions that have fluctuated over the past several years.
A complete lack of any official independent lab or field study showing the products chemical makeup or longer term effect on historic stone.
And some voice concerns about a product they fear may have some sort of sealant quality, in their eyes, because many find that water beads on the stone after this product is applied. And there for, they worry the product is interfering with wicking, water travel, and natural water displacement in and out of the stone.
The do no harm practice view is all about the use of products that have been tested and proven, as much as is possible, to best insure damage does not occur if used on historic stone. Wet & Forget has no such study at this time that we are aware of. On the positive side, this product does have several similar qualities in its chemical makeup that other “approved” biocide products have. But, without proper analyzation, it is hard to tell just how these chemical combinations actually interact with one another. This product should be analyzed and rigorously tested in much the same manner as D/2 Biological Solution was tested, to best insure it does not do harm. And so, we can neither condemn nor condone this product until such testing has been done. As always…we caution you not to use untested products, and to please use proven do no harm products. Why take chances?